Second law of thermodynamics
Let :
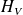 be the entropy of the body V
be the entropy of the body V
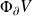 the flux of entropy through the surface
the flux of entropy through the surface
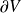 of V
of V
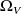 the supply of entropy to the body V
the supply of entropy to the body V
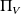 the production of entropy within the body V
the production of entropy within the body V
These quantities obey the balance law:
and it is the expression of the irreversibility of the physical processes that the entropy production
is non-negative for all processes obeying the conservation laws of mass, momentum, moment of momentum and energy as well as the material equations.





